DCAT Week
Fresenius Kabi, Formycon Launch Otulfi in U.S.
Otulfi was approved by the FDA for both subcutaneous and intravenous formulations as a biosimilar to Stelara.
Fresenius Kabi and Formycon AG have announced that Otulfi (ustekinumab-aauz), an ustekinumab biosimilar for the reference product Stelara (ustekinumab), developed by Formycon AG, is now available in the United States.
Otulfi is indicated for the treatment of moderately to severely active Crohn’s disease and ulcerative colitis in adult patients, moderate to severely plaque psoriasis for adult patients and pediatric patients six years or older, who are candidates for phototherapy or systemic therapy, and active psoriatic arthritis in adults and pediatric patients six years or older.
In February 2023, Fresenius and Formycon entered a global commercialization partnership for the ustekinumab biosimilar covering key global markets. The drug received FDA approval in September 2024.
“The U.S. availability of Otulfi demonstrates our commitment to serving patients and clinicians. Through the expansion of our biopharma portfolio, we are able to do this globally and in the U.S.,” said Dr. Sang-Jin Pak, President Biopharma and member of the Fresenius Kabi Management Board. “In addition to approving Otulfi last year for all indications matching the reference product Stelara, the FDA has also granted a provisional determination of interchangeability for Otulfi.”
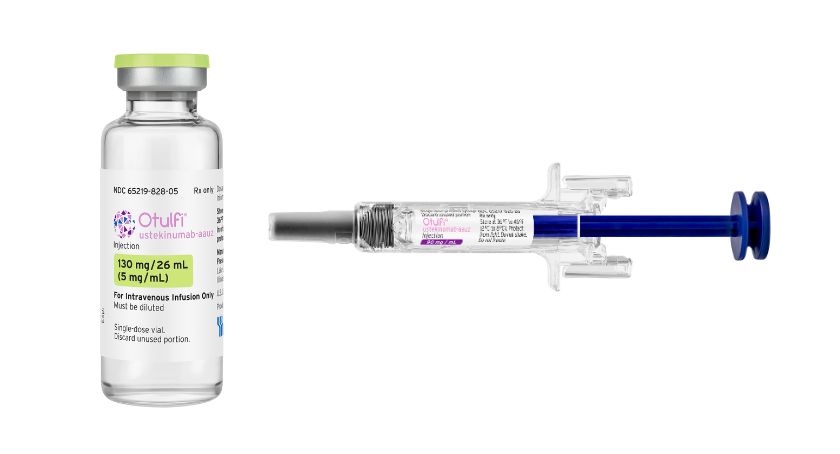
Otulfi will be available in the U.S. in the following presentations:
- a 45 mg/0.5 mL and 90 mg/mL single-dose prefilled syringe for injection
- a 130 mg/26 mL (5 mg/mL) single dose vial for IV infusion
Otulfi in a 45 mg/0.5 mL single-dose vial for subcutaneous injection is anticipated to receive FDA approval in the first half of 2025.



