BIO 2025
IL Group Exhibits Light Protection Solutions for Vials & Syringes
Its proprietary Light Protect Pack offers enhanced drug protection and clear content visibility.
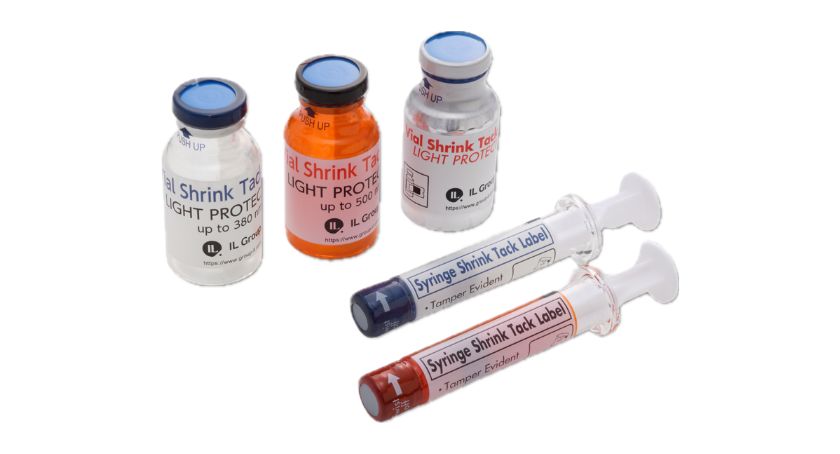
IL Group’s U.S. entity, Iwata Label USA, will highlight its proprietary Light Protect Pack, a multifunctional, light-shielding label solution that offers enhanced drug protection and clear content visibility for vials and syringes, at the BIO International Convention 2025.
Designed to replace conventional amber packaging, IL Group’s Light Protect Pack maintains drug stability by blocking light with wavelengths as low as 380 nanometers (violet) and with (amber) material as low as 500 nanometers, which are also available as part of the lineup. Unlike conventional light-protective solutions, IL Group’s labeling solution can successfully shield light even for applications using clear glass vials, or syringes (both glass and plastic) allowing visual inspection during the reconstitution process while still providing long-range light protection. This ensures the stability of light-sensitive pharmaceuticals from packaging to administration, enhancing both product safety and usability.
“Our solutions solve critical pain points for pharmaceutical manufacturers who are moving beyond the limitations of amber glass,” said Eiji Funabashi, President for Iwata Label USA Inc. “We’re excited to show how our technology improves both performance and flexibility in drug delivery systems.”
In addition to its advanced labeling technologies, IL Group will also highlight its contract packaging services in Japan, designed to help pharmaceutical companies streamline production and accelerate time to market. These services are tailored to meet the high standards of clinical trial, commercial, and specialty product packaging.



