Water purified by distillation or by reverse osmosis, so that it contains no added substance. WFI meets the purity requirements under Purified Water (USP). Although not intended to be sterile, it meets a test for a limit of bacterial endotoxin.
Glossary
Water For Injection (WFI)
02.27.12
-
Breaking News | Industry News

FUJIFILM Wako Introduces PYROSTAR® ES-F Line to U.S.
A line of reagents engineered to detect the presence of bacterial endotoxins.Contract Pharma Staff
-
Promotions & Moves
Akebia Therapeutics Names CMO
Jain to lead clinical development of the company’s HIF pipeline
-
Elemental Impurities: A Virtual Company Perspective
A look at risk assessments for elemental impurity determinations in oral tablet and parenteral drug products.Anthony DeStefano and Thomas Kester, Recordati Rare Diseases, Inc.
-
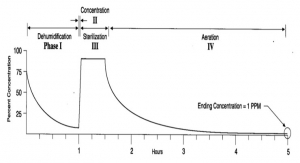
Cycle Parameters For Decontaminating A Biological Safety Cabinet Using H2O2 Vapor
Acumen White Papers - Volume 1 No. 2Released by: Baker
-
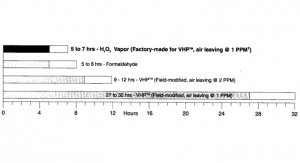
Using Hydrogen Peroxide Vapor To Decontaminate Biological Safety Cabinets
Acumen White Papers - Volume 1 No. 1Released by: Baker












