Live From Shows
-
CPHI North America

GRAM Expands Syringe and Cartridge Capacity
GRAM Expands Syringe and Cartridge Capacity
New 150,000 sq.-ft. syringe and cartridge filling center designed to hold up to four syringe/cartridge filling and inspection lines.
-
CPHI North America

"3 Key Trends" with Stuart Needlemen
"3 Key Trends" with Stuart Needlemen
Piramal Pharma Solutions’ Chief Patient Centricity Officer and Chief Commercial Officer offers his thought leadership.
-
CPHI North America

Collaboration and Partnering on Advanced Manufacturing Technologies
Collaboration and Partnering on Advanced Manufacturing Technologies
Kevin Webb from the API Innovation Center talks about achieving a domestic supply network for essential/critical medicines.
-
CPHI North America

Strategies for Navigating the Evolving Pharmaceutical Supply Chain Landscape
Strategies for Navigating the Evolving Pharmaceutical Supply Chain Landscape
Exploring the complexities and evolving trends within the API market with Jack Stine from Savendor.
-
CPHI North America

Sharp to Expand Its Macungie, PA Site
Sharp to Expand Its Macungie, PA Site
The site will double in size to increase its capacity for sterile injectables secondary packaging.
-
CPHI North America

CPHI North America 2024: Bringing Together Innovation in Pharma
CPHI North America 2024: Bringing Together Innovation in Pharma
Where the U.S. pharma market gathers.
-
CPHI North America

CPHI North America to Address Key Trends in Contract Services for 2024 & Beyond
CPHI North America to Address Key Trends in Contract Services for 2024 & Beyond
The event will see some 58 sessions with several notable keynotes.
-
CPHI North America

CPL Supports Customers with New Services
CPL Supports Customers with New Services
An integrated approach to topical drug development aims to save customers time and cost.
-
CPHI North America

"3 Key Trends" with Rob Feltz
"3 Key Trends" with Rob Feltz
Sharp Services’ Director of Analytical and Formulation Services offers his thought leadership.
-
CPHI North America

Adare Expands High Potency Handling Capabilities in the U.S. and Europe
Adare Expands High Potency Handling Capabilities in the U.S. and Europe
Announces facility expansions in Philadelphia, Vandalia and Milan.
-
CPHI North America

INCOG Manufacturing Facility Receives FDA Approval to Produce Drug Product
INCOG Manufacturing Facility Receives FDA Approval to Produce Drug Product
The approval comes after a rigorous inspection of INCOG's facility and quality systems.
-
CPHI North America

Kühne Holding AG to Acquire Aenova Group
Kühne Holding AG to Acquire Aenova Group
Enlarges its investment portfolio to include healthcare and pharmaceutical assets.
-
CPHI North America

Aurigene & Vipergen Partner to Co-Market & Offer DEL Screening Technologies to Customers
Aurigene & Vipergen Partner to Co-Market & Offer DEL Screening Technologies to Customers
Will also co-develop new DELs with an emphasis on novel chemical diversity and drug-likeness to further strengthen their service offering.
-
CPHI North America

CordenPharma, GENEPEP Celebrate R&D Alliance
CordenPharma, GENEPEP Celebrate R&D Alliance
Leverages peptide drug substance discovery and clinical development expertise.
-
CPHI North America

Kindeva, Syntegon Install First Versynta microBatch in North America
Kindeva, Syntegon Install First Versynta microBatch in North America
New Versynta microBatch underlines Kindeva’s injectable CDMO capabilities.
-
CPHI North America

CoreRx Completes Acquisition of Societal CDMO
CoreRx Completes Acquisition of Societal CDMO
Societal CDMO is now a wholly-owned subsidiary of CoreRx.
-
CPHI North America

Delpharm Acquires Astellas’ Meppel Solid Dosage Production Facilities
Delpharm Acquires Astellas’ Meppel Solid Dosage Production Facilities
The site produces more than 40 million finished products packaged annually.
-
Interphex 2023

KORSCH to Introduce Advanced X 5 Tablet Press at INTERPHEX
KORSCH to Introduce Advanced X 5 Tablet Press at INTERPHEX
Incorporates flexibility and innovation in a higher-speed, single-sided platform.
-
Interphex 2023
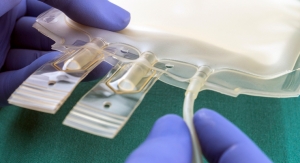
TekniPlex Healthcare to Debut Bioprocessing Solutions at INTERPHEX
TekniPlex Healthcare to Debut Bioprocessing Solutions at INTERPHEX
Will highlight its biologics portfolio of solutions across upstream, downstream processing and final fill operations.
-
DCAT Week
DCAT Week Unveils Best Practices for Bio/Pharma Supply Chain Optimization
DCAT Week Unveils Best Practices for Bio/Pharma Supply Chain Optimization
Debuts a new education program: "The Best Practices Platform: Industry Case Studies."












