FDA Watch
-
Analytical Services | Bioanalytical Services | Laboratory Testing | QA/QC

Considerations When Outsourcing Stability Testing
Stability studies are a critical element of drug development and a key commitment in maintaining product on the market.Paul Mason, Lachman Consultants 09.13.22
-
Information Technology | QA/QC | Regulatory Affairs

Data Governance for Outsourced Facilities Management Services
Data integrity is a growing and mainstream concern within the pharmaceutical industry.Scott Deckebach, Director & Patrick Day, Principal Consultant, Lachman Consultants 06.07.22
-
Biologics, Proteins, Vaccines | Regulatory Affairs

Phase-Appropriate cGMP Considerations for Cell and Gene Therapy
Readiness to begin GMP manufacturing is a decision point that requires careful consideration.Keith Lamb, Executive Director, Lachman Consultants 05.02.22
-
Information Technology | QA/QC | Regulatory Affairs

The Importance of Data Integrity Risk Assessment
Executing Data Integrity Risk Assessments for systems that generate and store both paper (manual)-based systems as well as computer-based and hybrid systems.Paul Mason, Ph.D., Lachman Consultants 06.07.21
-
Drug Delivery

The Ever-Evolving World of Combination Products
Looking at the benefits and challenges of the trend towards combination products.Ricki A. Chase, M.S, Lachman Consultants 04.01.21
-
Solid Dosage/Creams/Ointments
Recent Advances in Solid Dispersion Technologies
Improving the bioavailability and therapeutic efficiency of poorly soluble drugs.José L. Toro, Directory, Lachman Consultants 01.27.21
-

The Importance of Hypothesis Testing During Investigations
Why it is critical to have controls in place during out-of-specification investigations to provide quality assurance.Paul Mason, PhD, Lachman Consultants 11.17.20
-
Biologics, Proteins, Vaccines | Regulatory Affairs

Ensuring Manufacturing Continuity for Essential Medicines
Applying the principles of Quality Risk Management to get medicines to market faster.John Darby, M.Sc., Senior Director, Lachman Consultant Services, Inc. 09.09.20
-
Biologics, Proteins, Vaccines
Pharma’s Fight Against the Coronavirus Pandemic
A look at the pharma industry’s concerted effort to develop vaccines against the SARS-CoV-2 virus and therapeutics to treat COVID-19.Keith O. Webber, Lachman Consultant Services, Inc. 07.15.20
-
Information Technology | Regulatory Affairs

Data Integrity: Beyond Electronic Records
Get your Data Integrity house in orderTim Rhines, Ph.D., Director, Lachman Consultant Services, Inc. 05.05.20
-
Regulatory Affairs

Inquiring Minds Want to Know, Just Ask FDA
What you need to know prior to submissionMichelle Ryder, Lachman Consultant 04.01.20
-
Regulatory Affairs

The Art of Filing NDA/ANDA Post-Approval Changes to the FDA
Some tips to make your trip along the regulatory pathway to report manufacturing changes easier.Amy Schutte, Senior Associate, Lachman Consultant Services 03.04.20
-
Regulatory Affairs

Inactive Ingredients: Where Guidance Needs to Meet Data
Looking at FDA’s most recent draft guidance and the struggle with inactive ingredients.Sharif Ahmed, Lachman Consultants Services 10.15.19
-
Regulatory Affairs

Continuous Manufacturing and its Regulatory Challenge
...José L. Toro, PhD, Lachman Consultants 09.16.19
-
QA/QC | Validation
Considerations for Analytical Method Validation Lifecycle Controls
ICH is set to implement new regulatory guidance dedicated to analytical method developmentPaul Mason, PhD, Lachman Consultants 07.15.19
-
Regulatory Affairs
Effective Post Market Supplier Strategy for Combination Products
Combinations are typically designed, developed and maintained under a robust contract manufacturing processLori-Ann Woodard CBA, CQE, CSQE, Lachman Consultant Services 06.13.19
-
QA/QC

Nitrosamines
How to address these unwelcome guests in the pharmaceutical worldAloka Srinivasan, Ph.D., Lachman Consultant Services 05.07.19
-
Regulatory Affairs
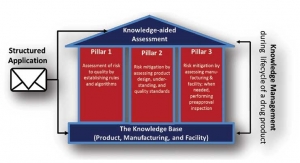
KASA to Support Generic Drug Review
FDA expects the new system to advance OPQ’s focus on pharmaceutical quality, the foundation for ensuring the safety and efficacy of drugs.Sharif Ahmed, Lachman Consultant Services 04.05.19
-
Regulatory Affairs

FDA’s Interpretation of the “Deemed to be a License” Provision of the Biologics Price Competition an
A look at how the FDA will administer the transition of NDAs to BLAsKeith Webber, Ph.D., Lachman Consultant Services 03.06.19
-
Regulatory Affairs

Reporting CPPs to FDA
Importance of identifying critical process parameters in original submissions to FDA.Aloka Srinivasan, Ph.D. Lachman, Consultant Services 10.10.18













